 Booster for Faster Pain Relief
Booster for Faster Pain Relief
Maximus Joint Booster is a booster joint supplement that aims to boost the healing effect of UC-II by combining with the world's patented herbal based anti-inflammation ingredient - Aflapin® (Boswellia serrata gum resin). Aflapin® is a novel synergistic composition derived from Boswellia serrata gum resin (Indian Patent Application No. 2229/CHE/2008). Aflapin is significantly better as an anti-inflammatory agent compared to the Boswellia extracts presently available in the market. A 90-day, double-blind, randomized, placebo-controlled study was conducted to evaluate the efficacy and tolerability in the treatment of osteoarthritis (OA) of the knee (Clinical trial registration number: ISRCTN80793440). At the end of the study, Aflapin conferred clinically and statistically significant improvements in pain scores and physical function scores in OA subjects. Interestingly, significant improvements in pain score and functional ability were recorded as early as 7 days after initiation of the study in the treatment group. Aflapin® is capable of inhibiting cartilage degrading enzyme MMP-3 and has the potential to regulate the inflammatory response by inhibiting ICAM-1.
Clinical Study on Aflapin® a novel synergistic composition derived from Boswellia serrata gum resin
 UC-II is sourced and manufactured in the United States. UC-II was determined GRAS in an intensive review of all safety and toxicology data by a panel of renowned United States scientific experts.
UC-II is sourced and manufactured in the United States. UC-II was determined GRAS in an intensive review of all safety and toxicology data by a panel of renowned United States scientific experts.
Derived from chicken sternum cartilage, UC-II® consists of undenatured type II collagen that supports joint comfort, flexibility and mobility.* UC-II is a FDA-notified and published new dietary ingredient. Supported by six animal clinical studies and backed by numerous patents, UC-II is the only source of undenatured type II collagen available as a powdered, shelf stable dietary ingredient.
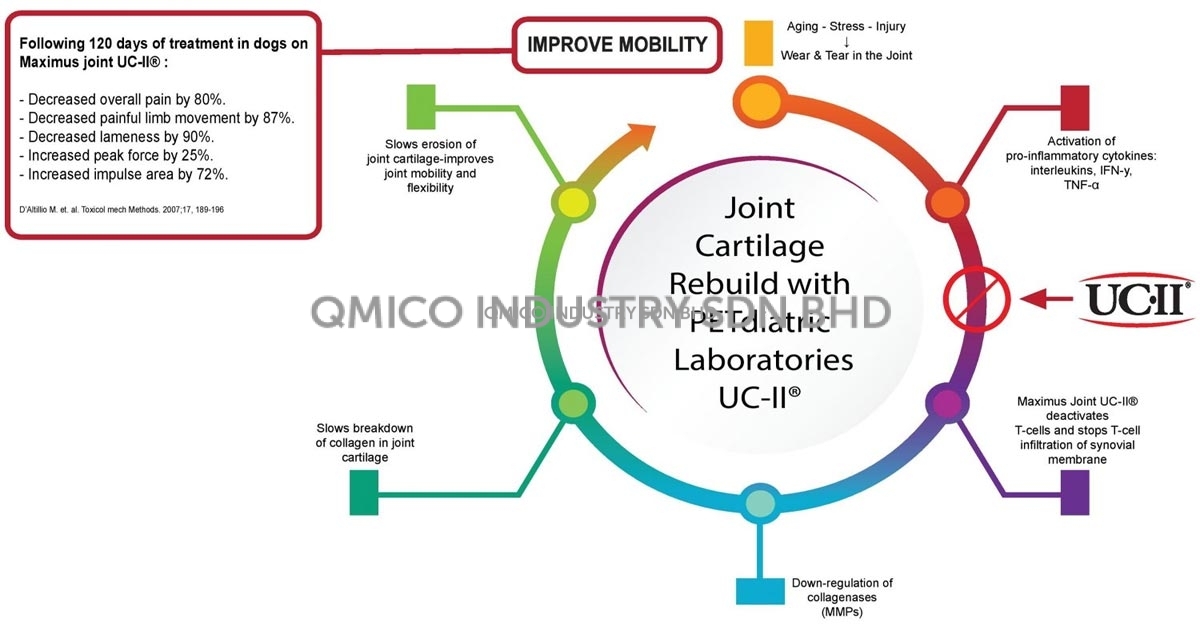
Maximus Joint® (UC-II) Helps Slow Down the Joint Destructive Process
Maximus Joint® (UC-II) Allows Normal Cartilage Remodeling to Begin for Continued Joint Comfort.
 Clinical Research
Clinical Research
Numerous research studies in humans and different animal species have been conducted and published. The research path of UC-II began when researchers, including Harvard University Medical Center, found the benefits of undenatured type II collagen for joint health and published research in peer-reviewed journals. Since then, InterHealth has continued to systematically and methodologically build the research portfolio for UC-II.
Clinical Study on UC-II® ;
Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis.
Efficacy and tolerability of UC-II as prevention against osteoarthritis.
Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee.
Comparison on efficacy of UC-II® compared to Glucosamine + Chondroitin on arthritic dog.
Better Joint Protection
UC-II, with clinical studies, shows to stop the host immune system from producing specific T-cells that attacks the joint.
Clinical Study on UC-II® ;
Pain reduction measured by ground force plate in arthritic dogs treated with type-II collagen.
Safety and toxicological evaluation of undenatured type II collagen.
X-ray with a comparison of bone structure since the time of the accident of a dog name - Chelsea, a Schnauzer.With UC-II® the bone structure is clearly redefined due to remineralization of the bone tissue.UC-II® is clearly proven in this case to stop joint degeneration,for treatment & prevention. 90% recovery in 150 days.


